On March 13, 2025, with the issuance of the first prescription by Dr. Chen Muqing in the Emergency Department and Trauma Center of Zhuhai People's Hospital, the world's first recombinant anti-tetanus toxin monoclonal antibody drug - Sintetol (generic name: Siltartoxatug Injection ) officially embarked on its journey to benefit patients in clinical practice.
This innovative biological anti-tetanus monoclonal antibody drug independently developed by Zhuhai Trinomab BioPharmaceutical Co., Ltd. (referred to as "Trinomab") has taken only 29 days from the date of marketing approval to the first clinical prescription, marking a major innovation in the field of tetanus prevention and treatment in China and promising to offer patients a new, superior and more efficient prevention and treatment option.

(Sintetol, the World's First New-Generation Tetanus Injection)
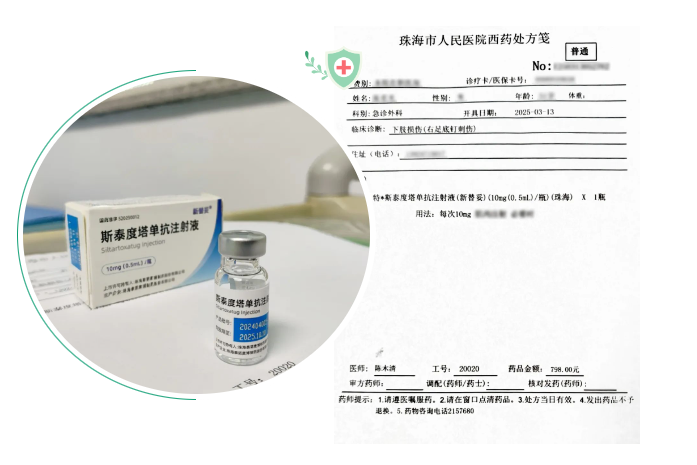
(Sintetol's First Prescription and Post-Administration Medication)
Revolutionary Breakthrough Achieved in Tetanus Prevention and Treatment
At the site of the first prescription issuance, Dr. Chen, Secretary of the Emergency Department and Trauma Center at Zhuhai People's Hospital, demonstrated the streamlined administration process of Sintetol to journalists:"Traditional passive tetanus immunizing agents, such as Tetanus Antitoxin (TAT) and Equine Tetanus Immunoglobulin F(ab')?, require complex procedures including skin testing, desensitization injections when skin testing as positive, and post-dose observation. In contrast, Sintetol derived from a native human monoclonal antibody reflecting natural anti-tetanus immunity and produced by recombination DNA antibody, eliminating the need for skin testing or dose adjustments. It truly achieves a 'one-shot solution', while eliminating the risk of anaphylactic shock or even death caused by false-negative skin test results!"
Phase III clinical trial data revealed that Sintetol rapidly reaches the protective levels of anti-tetanus toxin neutralizing antibody within 12 hours post-administration and maintains the effective antibody titers for a median duration of 132 days for better coverage for patients with longer tetanus incubation period[1].
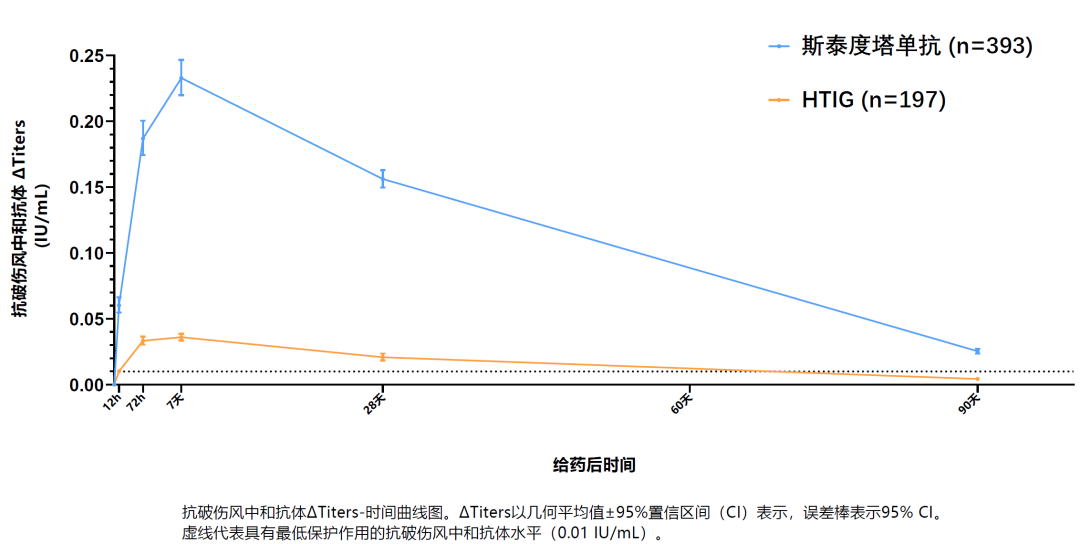
(Anti-Tetanus Neutralizing Antibody Levels (Geometric Mean ± 95% CI) in Phase III Trials)
Deadly Threat of the 'Silent Killer' Demands Innovative Solutions
According to the National Health Commission's Diagnosis and Treatment Guidelines for Non-Neonatal Tetanus (2024 Edition), tetanus remains a critical global public health threat. Without medical intervention, fatality rate is almost 100%. Even under modern medical care, the fatality rate globally remains alarmingly high at 30%–50%[2].
The current prevention and treatment system faces dual challenges:
Tetanus Antitoxin (TAT): Widely recognized as a "tetanus shot," TAT—a equine blood-derived product—carries significant allergy risks, including anaphylactic shock, serum sickness, or even death[3]. Many developed countries have phased out TAT due to these serious safety risks. Notably, the World Health Organization (WHO) removed TAT from the Essential Medicines List in 1991[4].
Human Tetanus Immunoglobulin (HTIG): HTIG preparations are produced from human plasma as raw material with limitation of short supply. It also poses potential risks of transmitting know or unknown blood-borne pathogens. Recently, China’s National Medical Products Administration (NMPA) mandated a update to all HTIG drug labels to include additional information that the product may cause allergic reaction and even serious allergic shock [5].

(Revisions to Adverse Reactions in HTIG Drug Labeling)
Biopharmaceutical Breakthrough Overcomes Threefold Challenges
During an interview, Zhao Wengui, General Manager of the Marketing Center at Trinomab, unveiled the technological breakthrough: "Trinomab’s independently developed Class I New Drug 'Sintetol' represents a disruptive innovation in tetanus prevention. As a first-in-class of anti-tetanus toxin monoclonal antibody, this product leverages globally leading fourth-generation antibody technology for discovery and development of native human monoclonal antibodies. Our intelligent and modular production systems have completely eliminated plasma dependency, thereby eradicating the risk of blood-borne pathogen transmission.
Clinical data demonstrate that a single intramuscular injection of Sintetol provides rapid immune protection with no need for skin testing, weight-based adjustment, or wound severity assessment. No post-injection observation is needed for outpatients, achieving true 'one-and-done' convenience. Importantly, Sintetol also delivers better protection for patients with long incubation periods, which might not be covered by the current existing therapies because incubation period of tetanus onset is ranging from<48 hours="" to="">30 days (30%). With production capacity reaching tens of millions of doses per year, Sintetol is poised to alleviate China’s supply pressures for passive immunization preparations."

(Scene of Sintetol Industrialization Production Workshop)
Government-Enterprise Collaboration Achieves "China Speed"
It is worth noting that time from marketing approval to the actual use of Sintetol was just 29 days. "This rapid progress is attributed to innovation and efficient coordination between Drug administration authorities in Guangdong Provincal and Zhuhai Municipal Levels," said Zhao Wengui, VP in commercial marketing of Trinomab Biopharmaceuticals. He emphasized that the company is accelerating the process for Sintetol under the nationwide medical insurance coverage, advancing commercial distribution strategies, and strengthening scientific education to ensure the drug reaches more healthcare institutions and patients across the country, fulfilling its mission to "create clinical value."
Zhu Ziqin, Party Committee Secretary and Director of the Zhuhai Municipal Health Commission, highlighted that Trinomab’s innovative drug Sintetol has made significant strides in addressing clinical challenges with traditional tetanus treatments, contributing to faster realization of universal health coverage.
Tian Hua, Deputy Director of the Zhuhai Municipal Science and Technology Bureau, indicated that the Bureau has introduced “Measures to build a highland for the transfer and transformation of scientific and technological achievements”, and taken a lead in incorporating application scenario innovation into the achievement transformation policy in the nation, and making every effort to create a "first city" for application scenario innovation and a highland for the transfer and transformation of scientific and technological achievements in the Guangdong-Hong Kong-Macao Greater Bay Area for scenario-driven innovation. Following the marketing approval, the first clinic use of Sintetol in the Zhuhai People’s Hospital was facilitated by the collaborative efforts between the Science and Technology Bureau and Health Commission. "We will continue to bridge technological innovation with market needs, fostering deeper integration of research and industry," the Deputy Director stated.
Cui Min, Party Committee Secretary of the Zhuhai People’s Hospital, reaffirmed the hospital’s commitment to patient-centered care and innovation. "We will persist in supporting new drug applications, aligning medical advancements with public health priorities to promote the development of new pharmaceutical productivity and improve healthcare accessibility," he added.

(Launch Ceremony Truck)
With the clinical application of Sintetol, China’s tetanus prevention and treatment system is poised for transformative upgrades Shifting from high-risk passive defense to precision-oriented proactive prevention, Transitioning away from blood dependent resources to industrial standardized recombination DNA antibody technology-driven solutions. This biological innovative drug developed under intellectual property rights owned by Trinomab is set to pioneer a new era for tetanus prevention and treatment worldwide.
References
[1]Sintetol Prescribing Information, NMPA Approval Document.
[2]Non-Neonatal Tetanus Diagnosis and Treatment Guidelines (2024 Edition), National Health Commission.
[3] Wang, C. Tetanus. People’s Medical Publishing House, 2022.
[4] MSF Medical Guidelines.
[5] National Medical Products Administration (NMPA). Announcement on the Revision of Package Inserts for Human Tetanus Immunoglobulin (No. 153 [2024]) [EB/OL]. (2024-12-24) [2025-03-10]. http://www.nmpa.gov.cn/xxgk/gzdt/202412/t20241224_153.html
(Sintetol Trademark Registration Number: 74174546)